Business Operation
Business Operation
Biolink helps with flu vaccine research
Influenza is an acute respiratory infectious disease caused by influenza virus. It is a highly infectious and fast spreading disease. According to the World Health Organization, flu affects about 1 billion people every year and can kill up to 500,000. Flu outbreaks affect us all, and quick and effective ways to diagnose, treat and prevent influenza must be found as soon as possible.
Help with flu vaccine research. Bieu is on the move. As a professional import company, bioulianke has been committed to solving the import problems of users in the biomedical industry since its establishment. The complex import process and strict requirements on temperature for special high-risk items such as viruses, host cells, antigens and antiserums related to influenza research are the strengths of Europa.

Every year, WHO predicts the preparation of influenza viruses for candidate vaccines. As a barometer for vaccine manufacturers, WHO distributes influenza viruses around the world through the UK's National Institute for Biological Control (NIBSC). The National Institute for Biological Standards and Control (NIBSC) is an international Standards supply center laboratory of WHO. Its core work is the preparation, preservation and distribution of WHO standards used to test the quality of biological products worldwide. NIBSC prepared influenza strains and detection antigens, antiserum standards, to ensure the smooth research and production of manufacturers.
NIBSC provides a wide range of international standard substances, reference materials and reagents for biomedical research. Its main products are as follows:
1. Biological standard substances, reagents supplied by AIDS Reagent Center and CJD Resource Center products
The NIBSC plays an important international role in preparing, evaluating and distributing international biological standards and other biological reference materials, providing more than 90% of these materials to the World Health Organization (WHO).
1.1 Biological standard substances include a large number of cytokines/growth factors, endotoxin, immunoglobulin and immune serum, protein hormones and endocrine factors, a large number of diagnostic reagents and vaccines, etc.

1.2 In addition to WHO standards, NIBSC has more than 2,000 reagents managed by the AIDS Reagent Center (CFAR).

1.3 The CJD Resource Centre provides characteristic materials for research and development of diagnostic tests for Creutzfeldt-Jakob disease (CJD).

2. Influenza products and biological drugs
The NIBSC's Influenza Resource Centre plays a major role in the production and standardization of influenza vaccines. The key problem with the development and production of influenza vaccines is that influenza viruses have a very high mutation rate, and the strains used for influenza vaccine development and production need to be updated at regular intervals. It played a key role in the production of WHO recommended vaccine and the selection of influenza strains. NIBSC provides customers with a wide range of the latest candidate influenza vaccines, viruses and related products. Through testing and evaluation, NIBSC scientists have helped to ensure the safety and effectiveness of biological products, while also helping to reduce the time it takes to bring new products to clinical use. New products, such as the latest generation of combination vaccines, need to be controlled in novel ways. The potential for a dramatic increase in the number and complexity of biological products, one of the effects of the genetic revolution, also requires more thorough control strategies.

3. The UK Stem Cell Bank supplies products and quality control serums for virus research
NIBSC provides a wide range of quality control serums for virus research as well as a variety of UKSCB human/mouse stem cell lines.
The attached:
The WHO web site:
https://www.who.int/
NIBSC released/product update site: https://www.nibsc.org/science_and_research/virology/influenza_resource_/full_reagent_update.aspx
NIBSC product enquiry website:
https://www.nibsc.org/products/brm_product_catalogue.aspx
NIBSC Public Mailbox:
standards@nibsc.org,can consult product technical problems.
NIBSC order hotline:010-84415639
How do I obtain NIBSC influenza standard
Q: How to query the strain number?
A: After WHO announced the flu strain, go to the NIBSC website to check the latest strain number published by NIBSC.
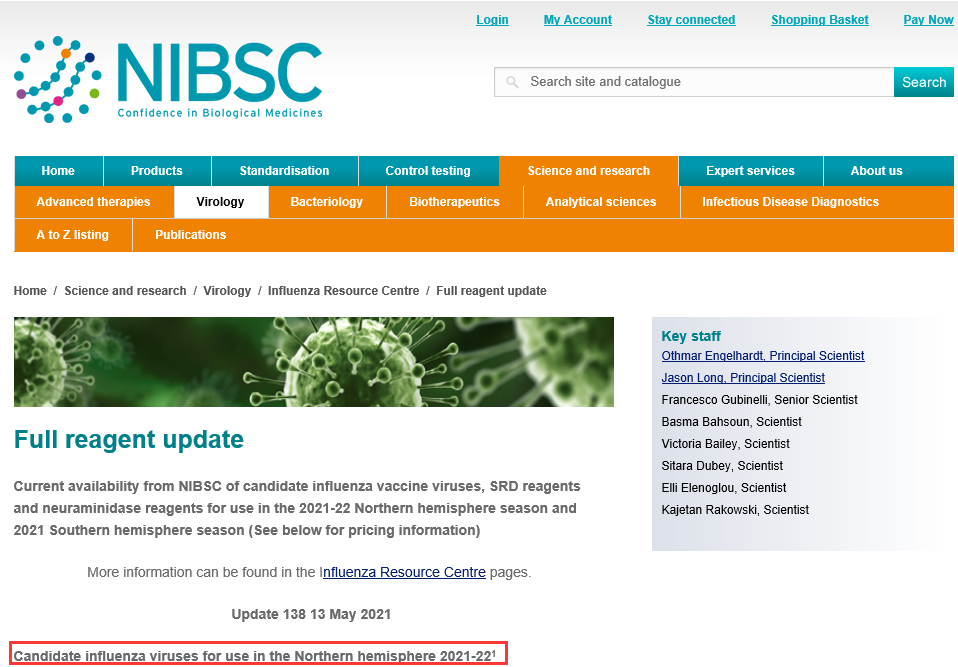
WHO website: https://www.who.int/
NIBSC released website: https://www.nibsc.org/science_and_research/virology/influenza_resource_/full_reagent_update.aspx
Q: What should I do if I cannot find the NIBSC website?
A: I can't find the website of NIBSC. Maybe NIBSC has not been prepared, or IT has been prepared, but its website has not been updated (IT is busy...) It can be found at NIBSC's product catalog website:
Enter English keywords in the search box and click "Search" to query.
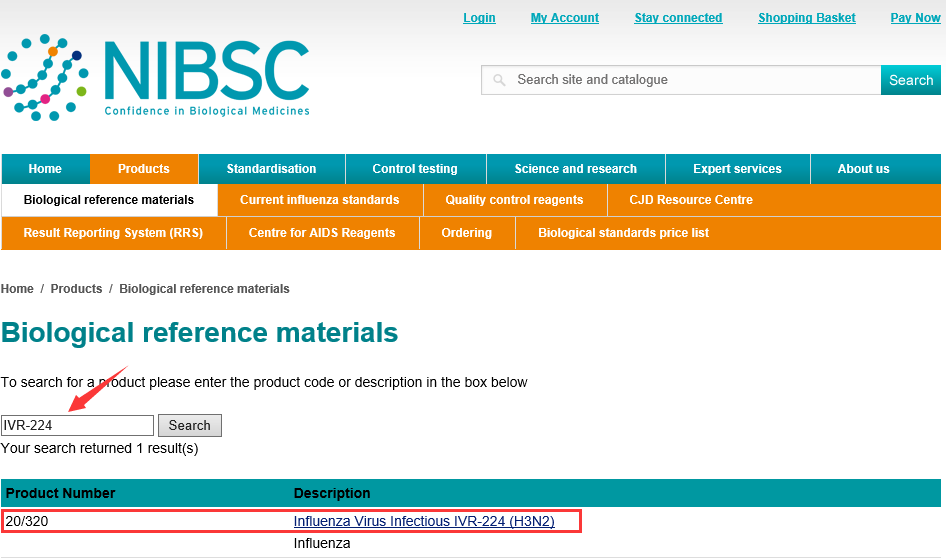
NIBSC catalogue website:https://www.nibsc.org/products/brm_product_catalogue.aspx
Q: How do I download the manual?
A: Log in to the WEBSITE of NIBSC product catalog, enter the article number (**/*** *) or English keywords in the search box, click the query result to enter the product information page, and then click the instruction file (**/***.pdf) to download.
Q: How do I order?
A: The company can provide comprehensive and efficient import, warehousing and logistics services. As NIBSC designated domestic foreign trade logistics business platform, we welcome you to order at any time.
Contact number: 010-84415639.
If you have any other questions about NIBSC, please leave a message and we will answer them as soon as possible.